Last Updated : Jul 14, 2020 10:00 AM IST | Source: Moneycontrol.com
FoMo or value buying? Smallcaps make quick recovery from March 24; 123 stocks rise over 100%
The BSE Smallcap index has rallied about 44 percent and the BSE Midcap index 35 percent since March 24. The Sensex has risen 37 percent and the Nifty 38 percent during the period.
Unwanted and ignored for two years, they have become the darling of the market over the last three months. The S&P BSE Smallcap index has vaulted 44 percent since March 24, when the benchmark indices hit their intermediate bottom, and more than 100 stocks have rallied over 100-800 percent during the period.
The rally in the smallcap space has been fast and furious. While many have stayed on the sidelines, several millennials as well as institutional investors have taken advantage of the volatility.
The S&P BSE Smallcap index has rallied by about 44 percent compared to 35 percent rally seen in the S&P BSE Midcap index. The S&P BSE Sensex has risen 37 percent while the Nifty50 is up 38 percent from March 24 lows.
Opto Circuits, Sintex Plastics, Reliance Power, IOL Chemicals, GTL Infrastructure and Reliance Home Finance are among 123 BSE Smallcap index stocks that have more than doubled investors’ money during the period.
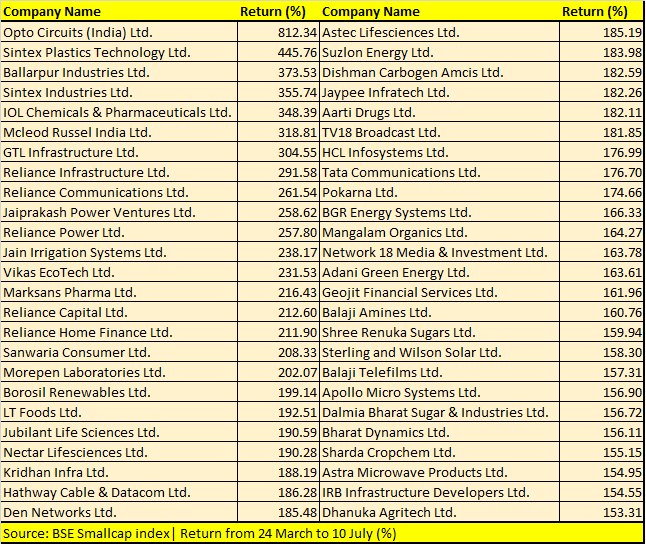
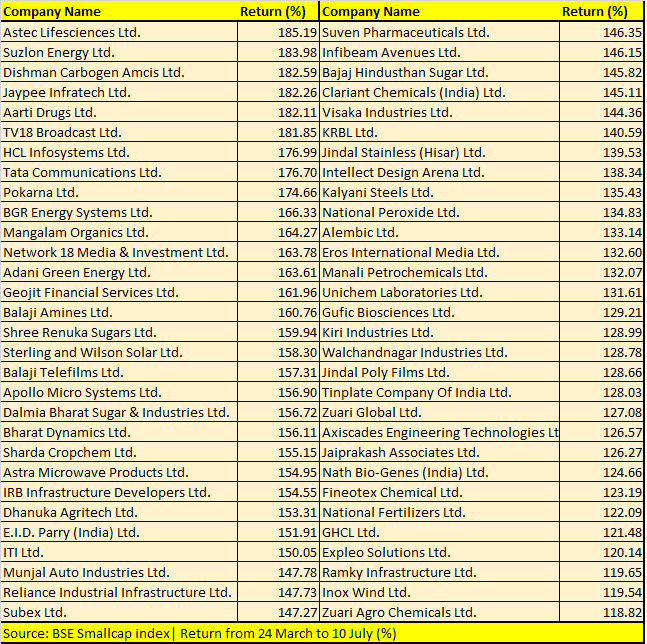
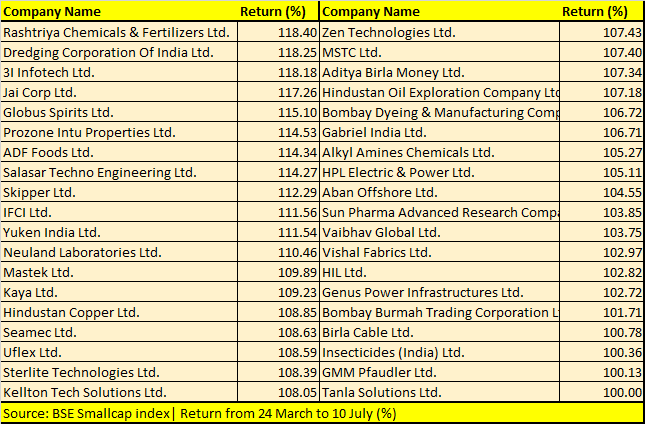
“We believe that the rally in the broader markets is a function of both institutional and retail interest. Given that the economy is on a mend, we are seeing increased investor interest in mid and smallcaps, given the beaten-down valuations that are driving the rally in broader markets,” Jyoti Roy, DVP Equity Strategist, Angel Broking Ltd, told Moneycontrol.
“While the rally so far has been driven by improving economic activity and liquidity, there one can build a case that the FOMO factor could be creeping into the markets, which may drive markets higher from current levels if global markets remain supportive,” he said, referring to the acronym for “fear of losing out”.
The mid and smallcaps have been in a correction since January 2018, when both the Nifty Midcap 100 and the Smallcap100 indices hit lifetime highs.
Since then, the midcap index has plunged 30 percent and the smallcap 50 percent as compared to the Nifty, which is trading lower in a single digit from its January 2018 levels.
“If you see, the smallcap index had a great 2017 and then everybody went into 2018 expecting a repeat. We all know how terrible 2018 turned out to be for smallcaps. Even 2019 was a poor year and then came 2020 March. At the lows of March 2020, the smallcap index had fallen nearly 50 percent from its highs of 2017,” said Shankar Sharma, co-founder, and vice-chairman, First Global.
“When and the index falls so much then you can take a reasonably calculated bet that it is going to outperform for the next few months, which is exactly what is happening.
What also happened in the last three years is that any number of smallcap companies became extremely undervalued. Most had been delivering decent performance but the market was not interested,” he said.
Disclaimer: The views and investment tips expressed by experts on Moneycontrol.com are their own and not those of the website or its management. Moneycontrol.com advises users to check with certified experts before taking any investment decisions.
First Published on Jul 14, 2020 10:00 am
TAGS #FOMO #Market Edge #MARKET OUTLOOK #Midcap #Portfolio Strategy #Sensex.Nifty #Smallcap #Stock Recommendations
Last Updated : Jul 14, 2020 11:27 AM IST | Source: Moneycontrol.com
Biocon to launch COVID-19 drug; stock jumps 5%
Biocon said the approval of Itolizumab, from the DCGI, is based on the results from the successful conclusion of a randomized, controlled clinical trial at multiple hospitals in Mumbai and New Delhi.
Moneycontrol News @moneycontrolcom
Shares of Biocon jumped almost 5 percent intraday on July 14 after the company said it will launch biologic drug Itolizumab for the treatment of moderate to severe COVID-19 patients at a price of around Rs 8,000 per vial.
The company has received approval from the Drugs Controller General of India (DCGI) to market Itolizumab injection 25mg/5mL solution for emergency use in India for the treatment of cytokine release syndrome in moderate to severe acute respiratory distress syndrome (ARDS) due to COVID-19.
Itolizumab will be manufactured and formulated as an intravenous injection at Biocon’s bio-manufacturing facility at Biocon Park, Bengaluru, said the company.
Biocon said the approval of Itolizumab, from the DCGI, is based on the results from the successful conclusion of a randomized, controlled clinical trial at multiple hospitals in Mumbai and New Delhi.
The study focussed on the safety and efficacy of Itolizumab in preventing CRS in moderate to severe ARDS patients due to COVID-19. The primary endpoints for the reduction in mortality rate were met and other key secondary endpoints for efficacy and biomarkers were also achieved, the company said.
“Itolizumab’s unique mechanism of action made it an ideal candidate for treating the ‘cytokine storm’, which is a leading cause of death in COVID-19 patients. I am pleased that our R&D and clinical teams delivered on this promising hypothesis in such a short period of time. It is a proud moment for all of us at Biocon and we would like more and more patients to benefit from this therapy," said Kiran Mazumdar-Shaw, Executive Chairperson, Biocon.
Shares of the company traded 3.17 percent up at Rs 427.75 on BSE around 11:10 hours. If the stock ends in the green, it will be its fifth consecutive session of gains.
First Published on Jul 14, 2020 11:27 am
TAGS #Biocon #Buzzing Stocks
Last Updated : Jul 14, 2020 11:23 AM IST | Source: Moneycontrol.com
Robinhood raises another $320 million, increasing its valuation to $8.6 billion
The additional money comes from investors like TSG Consumer Partners and IVP, helping Robinhood raise a total of USD 600 million in its seed round within two months.
Moneycontrol News @moneycontrolcom
US-based financial services company Robinhood said that it has raised an additional USD 320 million more to the previously-raised USD 280 million in its Series F funding round. The amount was raised at a total valuation of USD 8.6 million.
The additional money comes from investors like TSG Consumer Partners and IVP, helping Robinhood raise a total of USD 600 million in its seed round within two months.
Prior to the latest funding, Robinhood had announced in May that it had raised a total of USD 280 million, making the fintech company worth USD 8.3 million, reported TechCrunch.
The funding round is widely being seen as a precursor to an initial public offering (IPO), which has benefited from a surge in day trading, driven by consumers stuck at home during the coronavirus pandemic.
Robinhood has been a hit since its launch in 2015. The firm has reportedly added millions of funded accounts in 2020, as investors of all sizes take part in the year’s huge equity volatility due to the COVID-19 pandemic.
However, the Menlo Park, California-based startup has also experienced several outages on its app since early March, particularly on days of high trading volumes.
First Published on Jul 14, 2020 11:23 am
Last Updated : Jul 14, 2020 11:11 AM IST | Source: PTI
Cipla gets USFDA nod for rare genetic condition treatment drug
Cipla's Icatibant injectable pre-filled syringe in the strength of 30mg/3mL is generic version of Shire's Firazyr, the company said in a regulatory filing.
PTI
Drug major Cipla on Tuesday said that it has received final approval from the United States Food and Drug Administration (USFDA) for Icatibant Injectable, indicated for treatment of acute attacks of hereditary angioedema - a rare genetic condition - in adults.
Cipla's Icatibant injectable pre-filled syringe in the strength of 30mg/3mL is generic version of Shire's Firazyr, the company said in a regulatory filing.
The firm said "it has received final approval for its abbreviated new drug application for Icatibant Injectable 30mg/3mL from the United States Food and Drug Administration".
Quoting IQVIA (IMS Health) data, Cipla said Firazyr and its generic equivalents had US sales of approximately USD 270 million for the 12-month period ending May 2020.
Shares of Cipla were trading 0.49 percent higher at Rs 641.25 apiece on the BSE.
First Published on Jul 14, 2020 11:05 am
Smallcap index has rallied about 44 percent and the BSE Midcap index 35 percent since March 24. The Sensex has risen 37 percent and the Nifty 38 percent during the period.
https://www.moneycontrol.com/news/business/markets/fomo-or-value-buying-smallcaps-make-quick-recovery-from-march-24-123-stocks-rise-over-100-5541281.html












No comments:
Post a Comment